Abemaciclib is an oral, selective inhibitor of CDK4/6 that is dosed on a twice-daily, continuous schedule. In patients with hormone receptor (HR)-positive, HER2-negative advanced breast cancer (ABC), abemaciclib has demonstrated clinical efficacy with a tolerable safety profile when administered as monotherapy in MONARCH 1, in combination with fulvestrant in MONARCH 2, and in combination with nonsteroidal aromatase inhibitors in MONARCH 3.1-3 Inducing tumor response and delaying disease progression is of critical need in patients with liver metastases. Thus, an exploratory subgroup analysis was conducted in patients with liver metastases at baseline across the MONARCH 1, 2, and 3 studies.4 All patients had HR-positive, HER2-negative ABC. The primary end point of MONARCH 1 was objective response rate (ORR), and the primary end point of MONARCH 2 and 3 was investigator-assessed progression-free survival (PFS).
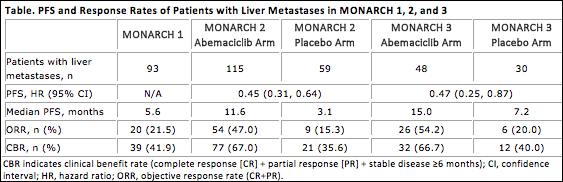
Efficacy results of patients with liver metastases are shown in the Table. The most frequent adverse events observed in patients with liver metastases in MONARCH 1 were diarrhea, nausea, and fatigue; in the abemaciclib arms of MONARCH 2 and 3, they were diarrhea, neutropenia, and nausea.
The authors concluded that the combination of abemaciclib plus endocrine therapy is an effective treatment option in patients with HR-positive, HER2-negative ABC with liver metastases, with PFS, ORR, and clinical benefit rate significantly higher than that derived from single-agent endocrine therapy. Tolerability results were generally consistent with the safety populations previously reported for each study.
References- Dickler MN, et al. Clin Cancer Res. 2017;23:5218-5224.
- Sledge GW Jr, et al. J Clin Oncol. 2017;35:2875-2884.
- Goetz MP, et al. J Clin Oncol. 2017;35:3638-3646.
- Di Leo A, et al. SABCS 2017. Abstract P5-21-02.