National Comprehensive Cancer Network (NCCN) guidelines recommend that premenopausal women with hormone receptor (HR)-positive, HER2-negative metastatic breast cancer (MBC) be rendered postmenopausal and then treated accordingly.1 The CDK4/6 inhibitor palbociclib, in combination with endocrine therapy (ET), has become a standard of care in the first-line or pretreated settings for women with HR-positive, HER2-negative MBC. Specialty pharmacy prescription data indicate that 14% of all women with HR-positive, HER2-negative MBC treated with palbociclib in the United States are aged younger than 50 years. In their presentation at SABCS 2017, the authors assessed the real-world treatment patterns and outcomes before and after approval of palbociclib in women with HR-positive, HER2-negative MBC.2 They further sought to assess the impact of the NCCN guidelines for premenopausal women on treatment patterns and outcomes.
This was a retrospective cohort study utilizing electronic health record (EHR) data from Flatiron Health (Fl) from January 2011 through March 2017 to evaluate patient characteristics and first-line ET treatment patterns among women with HR-positive, HER2-negative MBC prior to and after palbociclib approval in February 2015. Menopausal status was defined by age (≤50 years vs >50 years). Additional data sets of >13,000 patients with MBC in the Truven Health MarketScan and Optum Clinformatics claims and Humedica EHR databases were included to represent a more comprehensive data set and evaluate clinical outcomes.
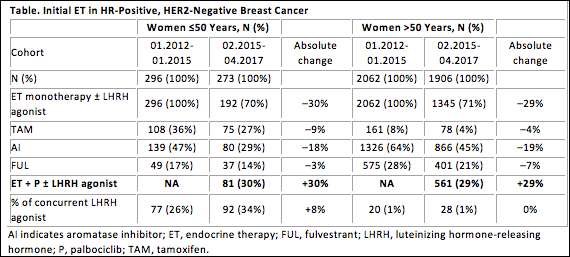
Initial results included 4537 patients in the FI database who initiated a first-line ET regimen. Overall, 30% of women ≤50 years used palbociclib, which is comparable to the proportion of women aged >50 years (27%) who received palbociclib. Treatment patterns for initial ET are shown in the Table.
Decreased use of tamoxifen as first-line ET was noted in patients aged ≤50 years over the observed time. About 46% of young patients initiated endocrine-based treatment with aromatase inhibitor monotherapy in the pre-palbociclib era, consistent with the NCCN guidelines. In the post-palbociclib era, use of endocrine-based treatment decreased to 23% in these patients. This decrease was offset by the increase in use of aromatase inhibitor‒palbociclib combinations.
The treatment paradigm for women with HR-positive, HER2-negative MBC has evolved over the past 5-plus years. Consistent with NCCN guidelines, more young patients are receiving treatment with aromatase inhibitors or fulvestrant in combination with palbociclib (with ovarian suppression as clinically indicated) as part of initial therapy. There has been a related decrease in use of tamoxifen for younger patients and overall. Palbociclib has been incorporated into standard care for US patients with HR-positive, HER2-negative MBC, regardless of age.
References- NCCN Guidelines. Breast Cancer. 2017. www.nccn.org/professionals/physician_gls/default.aspx#site.
- Burstein HJ, et al. SABCS 2017. Abstract P3-11-01.